
Sustainability
" Quality is real performance, not a promise "
Our Approach – GazMin Medi pharma objective is to establish novel market segments within our existing markets, in order to maintain a robust and inventive position. Our goal is to deploy our distinctive business model worldwide, ensuring inexpensive access to high-quality pharmaceuticals. At GazMin Medi pharma, we prioritize reliability and excellence in our products. Our medicines originate from recognized pharmaceutical producers and are compliant with WHO pharmaceutical guidelines and industry standards. You can trust us to deliver top-notch pharmaceutical solutions that meet your exact specifications.
" Achieving success requires a culture of discipline "
Excellent, Reliable Outsourced Manufacturing – GMP has established enduring and exclusive collaborations with prominent pharmaceutical groups and authorized distributers from India, China, Taiwan, Bangladesh, Korea, Eastern Europe, Israel and other nations. These major branded generic and branded pharmaceuticals and medical disposibles producers. All those products are certified under manufacturing facilities such as FDA, US-FDA CFDA, EU-GMP, MHRA, ANVISA, COFEPRIS and more. The majority of our products both generics and branded generics are WHO norms and standards for pharmaceuticals guidelines.

Join GazMin Medi pharma
Your gateway to a dynamic and rewarding career in global project management consultancy. At GazMin International, we believe in fostering a culture of excellence, innovation and collaboration. Join us on this exciting journey where your skills, aspirations and dedication converge to shape the future of project management on a global scale.



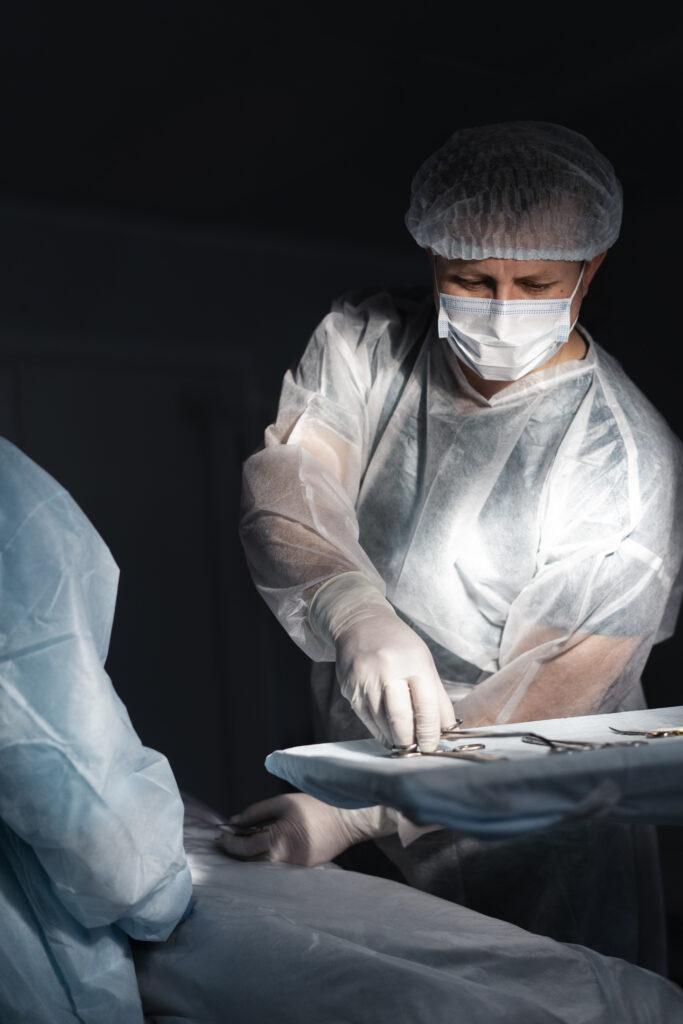
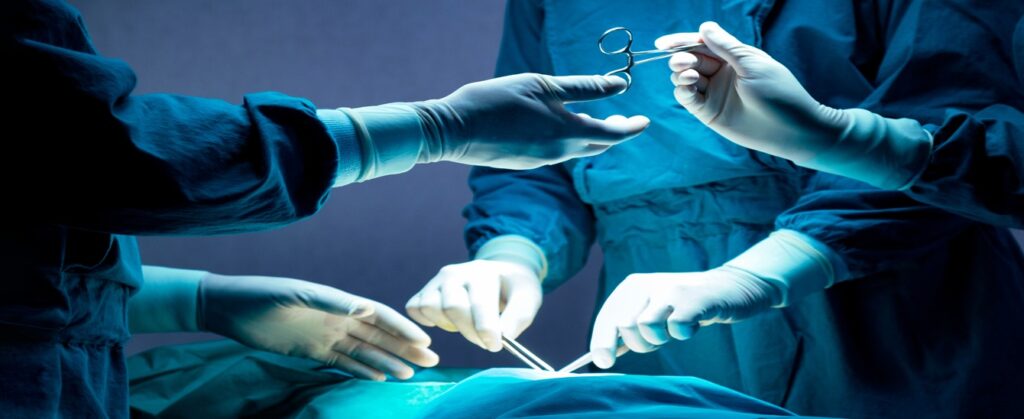
Reliable and effective services – When it comes to healthcare, we get the sense of urgency. When you work with us, you can anticipate service that is second to none. We have an extensive network of suppliers and logistics partners that allow us to deliver products quickly to any location worldwide. Our dedicated customer support team is available to address any inquiries or resolve any concerns you may encounter.
Our Code of Conduct – As a responsible pharmaceutical company, we strictly follow all regulatory requirements and recommendations. Our goods go through rigorous quality control methods to assure their safety and efficacy. You can rest assured that you are collaborating with an ethical and reliable partner.
The first objective of every medical professional is to ensure the health and well-being of their patients. we collaborate with organizations worldwide and maintain relations with virtually every country in order to tackle some of the most formidable global health challenges. Through establishing strong partnerships with pharmaceutical organizations and authorized distributors, we can collectively contribute to the improvement of safety, outcomes, cost-effectiveness, and accessibility to healthcare.
Moreover, during periods of global health emergencies, such as pandemics or outbreaks, a recognized pharmaceutical corporation assumes the duty of manufacturing and disseminating crucial protective medications. They have an essential role in protecting healthcare workers and individuals from any potential risks.
Our export strategy
Healthcare enterprises operating in heavily regulated sectors, such as the pharmaceutical industry. In addition to the customary export documentation. organizations must be cognizant of the specific documents necessary for their respective industries. These agreements guarantee the different facets of product quality standard, production procedures, and adherence to regulations in the sector in order to ship goods globally.
We ensure that our clients hold the necessary legal documentation in order to distribute and market their products internationally. Based on our extensive with multinational pharmaceutical companies, the following documents are crucial:
List of documents:
1) Certificate of Pharmaceutical Product (CPP)
2) Good Manufacturing Practice Certificates (GMP)
3) Export Shipping Documents
4) Free Sale Certificates
5) Health Products Regulatory Authority Certificates (HPRA)
6) Manufacturing Licenses
7) Certificates of Origin
8) Bureau of Indian Standards Certificates (BIS)
9) Veterinary Medicines Directorate Certificates (VMD)
10) European Medicines Agency Commission Reports (EMA)
These documents are essential for the lawful export of products. They contain vital information regarding the product, its intended destination, and the specifics of transportation, which greatly assist in the seamless process of customs clearance.

Reputed, Resourceful and Trusted supplier for an array of Medical Tools, Supplies and Equipment with state of the art technology.
Official Info
- 1503 & 1504 Grosvenor Business Tower, Business Bay, Dubai - U.A.E
- +971 4240 7778
- +971 4835 0458
- healthcare@gazminintl.com
